Jack Falcón, José Antonio Muñoz-Cueto. 2024. The pineal and reproduction of teleosts and other fishes. Chapter 9 In Hormones and Reproduction of Vertebrates, Volume 1 (Second Edition). Editors: David O. Norris, Kristin H. Lopez. Academic Press, pp 221-269. ISBN 9780443160097. https://doi.org/10.1016/B978-0-443-16009-7.00008-6
Contact BOREA : Jack Falcón , j.falcon-pro@orange.fr ou jack.falcon@mnhn.fr
Légendes des illustrations en galerie d'images (ci-dessous) :
Les activités rythmiques des poissons - dont la reproduction - sont synchronisées par les variations de l'environnement. Cela implique souvent des horloges biologiques circadiennes et circannuelles qui permettent l’anticipation. La photopériode est le signal environnemental le plus puissant utilisé par les organismes pour synchroniser leurs activités rythmiques. Bien que moins fiables, les variations cycliques de la température sont aussi à prendre en compte, car les poissons dépendent de la température externe pour ajuster leur température interne ; ceci affecte leurs processus métaboliques et physiologiques. L'organe pinéal joue un rôle clé dans le contrôle des rythmes quotidiens et annuels. Nous discutons ici l’ensemble des éléments qui constituent la base du contrôle photo- et thermo-périodique de la reproduction chez les poissons : (i) la place de l'organe pinéal dans le système circadien, son organisation morpho-fonctionnelle, les modalités de l'intégration des informations photo- et thermo-périodiques par ses cellules photoréceptrices, et la production subséquente de messages rythmiques, nerveux et neuroendocrines, dont la mélatonine, (ii) les voies permettant à ces messagers d’atteindre l'axe cerveau/hypophyse/gonades (CHG) via, d’une part, une innervation pinéalofuge et, d’autre part, la mélatonine et ses récepteurs, (iii) les données rapportant le rôle de la mélatonine dans le contrôle des productions journalières et saisonnières des hormones impliquées dans la reproduction à différents niveaux de l'axe CHG (dont : les gonadotrophines hypophysaires [FSH, LH], la thyréostimuline [TSH] et les stéroïdes sexuels). Nous proposons également des pistes de recherches qui devront tenir compte de ce que différentes modalités de ce contrôle existent chez les poissons, un groupe de loin le plus grand et diversifié de tous les représentants de vertébrés, et exposé à une grande variété de conditions et défis environnementaux.
Contact BOREA : Jack Falcón , j.falcon-pro@orange.fr ou jack.falcon@mnhn.fr
Légendes des illustrations en galerie d'images (ci-dessous) :
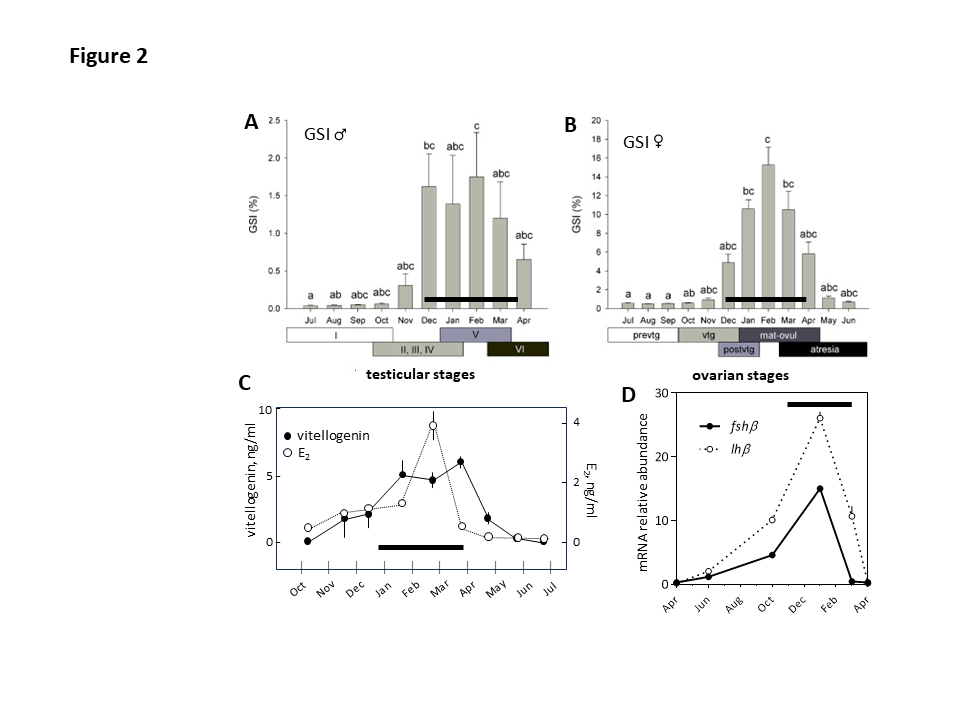
- Fig. 2 : Annual rhythm of reproduction in the European sea bass, Dicentrarchus labrax. Seasonal variations of the gonadosomatic index (GSI) in males and females (A, B), plasma vitellogenin and 17b-estradiol (E2) (C), and pituitary fshb and lhb mRNA abundance (D). The black bars correspond to the reproductive phase of the annual cycle. In A the stages are: I, immature; II, early recrudescence, III, mid-recrudescence; IV, late recrudescence; V, full spermiating; VI, post-spawning. In B the stages are: Females: prevtg, previtellogenesis; evtg (n = 10), early vitellogenesis; lat-postvtg, late-post-vitellogenesis; mat-ovul, maturation-ovulation; atre, atresia. Modified and adapted from (Falcón et al., 2021; Navas et al., 1998; Rocha et al., 2009). © rightLinks Elsevier - Number 5903761097626
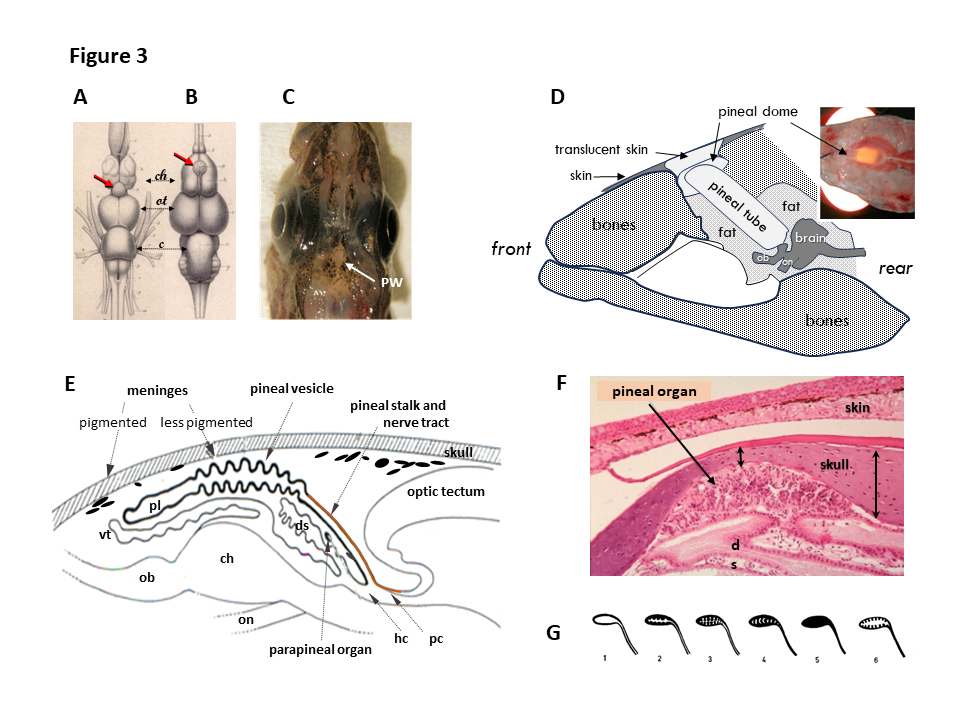
- Fig 3 : Anatomie de l’organe pinéeal (épiphyse) des poissons / Anatomy of the fish pineal organ. A, B. Dorsal view of the brains of the conger, Conger vulgaris (A) and stripped red mullet, Mullus surmulletus (B) showing the central location of the pineal organ (red arrows) above the cerebral hemispheres (ch). ot, olfactive bulb; c, cerebellum. Modified from Baudelot (Baudelot, 1883). C. Dorsal view of the head of the Arctic cod (Boreogadus saida) showing the pineal window (PW), an area where the meninges are less pigmented, centrally located in between the two lateral eyes (modified from Falcón et al., 2011). D. Schematic drawing of a sagittal section through the head of the bluefin tuna, Thunnus thynnus. In this species, the brain is located deep into the head, and a translucent cartilaginous structure (the pineal tube) allows light to reach the pineal gland. This pineal tube is covered by a translucent cartilage, the pineal dome, covered itself by thin translucent and less pigmented skin (drawing from personal observations and modified from (Rivas, 1953)). E. Schematic drawing of a sagittal section through the brain of the northern pike Esox lucius. The pineal vesicle is connected to the brain between the habenula (hc) and posterior (pc) commissures by the pineal stalk. The large pineal vesicle covers the cerebral hemispheres (ch) and olfactory bulbs (ob). The pineal lumen (pl), filled with cerebrospinal fluid, opens into the 3rd ventricle. ds, dorsal sac; on, optic nerve; vt, velum transversum. Modified from (Falcón et al., 2011). F. Eosin staining of a sagittal section through pineal area of the gilthead seabream, Sparus aurata. The pineal tissue is located in a pit, formed by the bone skull becoming substantially thinner in this area. G. Diagrammatic presentation of the 6 types of pineal epithelia in teleosts: 1, flat; 2: folded; 3, convoluted; 4, small space; 5, compact; 6, intermediate. Modified from (Omura & Oguri, 1969). © rightLinks Elsevier - Number 5903761097626
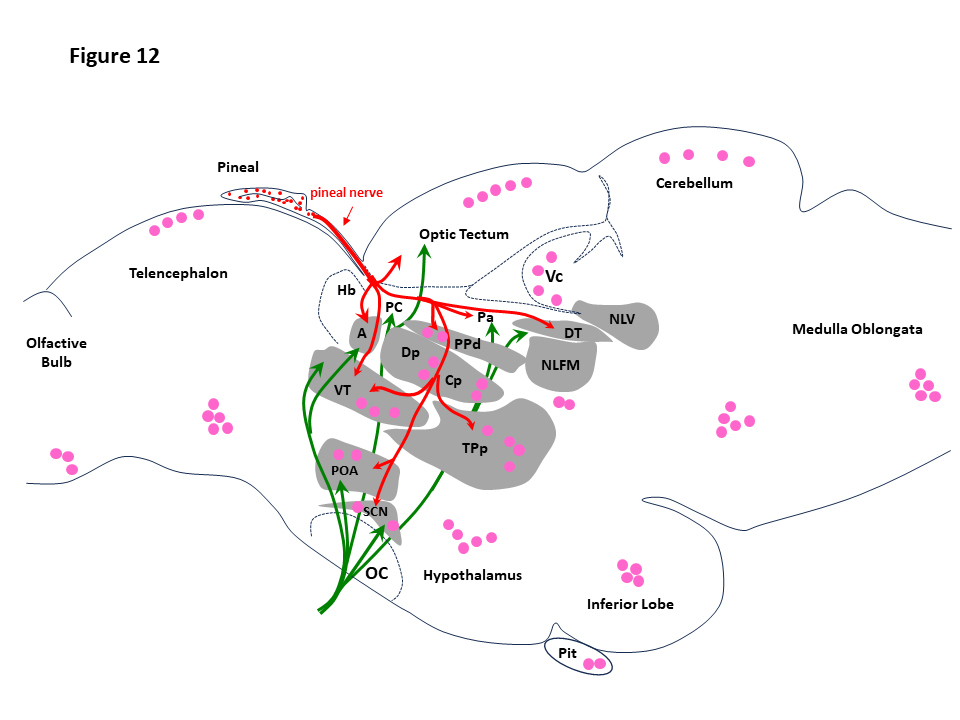
- Fig. 12. Pineal and retinal targets in the fish brain. The drawing is a schematic presentation of the pineal (red arrows) and optic (green arrows) nerve projections, together with the areas where the melatonin receptors have been identified (pink dots). A, anterior prethalamic nucleus; Cp, central posterior thalamic nucleus; Dp, dorsal posterior thalamic nucleus; DT, dorsal tegmental nucleus; Hb, habenula; NFLM, nucleus of the medial longitudinal fascicle; NG, nucleus glomerulosus; NLV, lateral nucleus of the valvula; OC, optic chiasm; OT, optic tectum; Pa, paracommissural pretectal nucleus; PC, posterior commissure; PPd, dorsal periventricular pretectal nucleus; POA, preoptic nucleus; RF, reticular formation; SCN, suprachiasmatic nucleus; TPp, periventricular nucleus of the posterior tubercle; Vm, trigeminal motor nucleus; TS, torus semicircularis; VT, ventral prethalamus. Data from (Yáñez et al., 2009 and Servili et al., 2011) for the pineal projections, and from (Herrera-Pérez et al., 2010; Mazurais et al., 1999, and Feng et al., 2019) for the melatonin receptors. © rightLinks Elsevier - Number 5903761097626
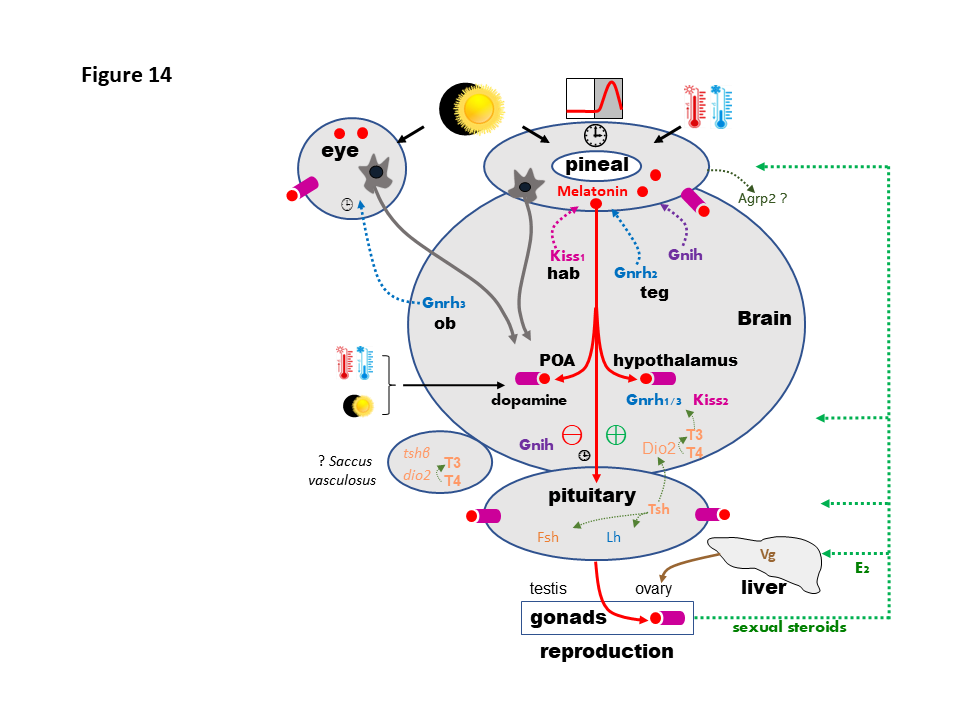
- Fig.14 : Schematic presentation of the photo- and thermo-periodic regulation of the brain-pituitary-gonadal (BPG) axis. The photoperiodic (retina and pineal) and photo-thermo-periodic (pineal) information reach the neuroendocrine axis through non-visual retinal and pineal nerve fibers (brown arrows) and pineal melatonin (red circles) released into the cerebrospinal fluid and circulation. Melatonin acts through specific receptors (purple boxes) found at all stages of the BPG axis. Deep brain thermoreceptors and photoreceptors might also operate in the basal telencephalon. Melatonin effects have been reported on the main preoptic (POA) and hypothalamic stimulatory (GnRH1/3, Kp2, ⊕) and inhibitory (GnIH, dopamine, ⊖) factors known to control fish pituitary gonadotropes (FSH and LH cells). At the pituitary level melatonin also modulates the production of adenohypophysial hormones, including those involved in the control of reproduction, FSH, LH and TSH. TSH from the pituitary may regulate FSH and LH productions via the pituitary folliculostellate cells and/or the brain deiodinase-2 (Dio2), which allows production of T3 (triiodothyronine) from T4 (thyroxine). FSH and LH act on the gonads (ovary and testis) to control the progress of gametogenesis and steroidogenesis. A feedback of gonadal steroids operates on the pineal gland, reproductive brain and pituitary (green dotted lines). Estradiol (E2) also acts on the liver of female fish to promote the synthesis and release of vitellogenin (Vg), which reaches the ovary through the vascular system and contributes to the progression of vitellogenesis. Moreover, nerve projections (colored dotted lines) from the habenula (hab), containing Kp1-producing cells, and mesencephalic tegmentum (teg), containing Gnrh2 and Gnih neurons, reach the fish pineal gland, whereas the retina receives Gnrh3 fibers originating from cells in the olfactory bulbs (ob). The saccus vasculosus (SV), present in some fish species, contains opsin proteins, Tsh and Dio2; it might also act as a sensor of seasonal changes in day length in some fish species. The pineal gland also produces AgRP2 (agouti-related peptide-2), although its function remains unknown. [⏱️] : circadian clock machinery. © rightLinks Elsevier - Number 5903761097626
- Fig 3 : Anatomie de l’organe pinéeal (épiphyse) des poissons / Anatomy of the fish pineal organ. A, B. Dorsal view of the brains of the conger, Conger vulgaris (A) and stripped red mullet, Mullus surmulletus (B) showing the central location of the pineal organ (red arrows) above the cerebral hemispheres (ch). ot, olfactive bulb; c, cerebellum. Modified from Baudelot (Baudelot, 1883). C. Dorsal view of the head of the Arctic cod (Boreogadus saida) showing the pineal window (PW), an area where the meninges are less pigmented, centrally located in between the two lateral eyes (modified from Falcón et al., 2011). D. Schematic drawing of a sagittal section through the head of the bluefin tuna, Thunnus thynnus. In this species, the brain is located deep into the head, and a translucent cartilaginous structure (the pineal tube) allows light to reach the pineal gland. This pineal tube is covered by a translucent cartilage, the pineal dome, covered itself by thin translucent and less pigmented skin (drawing from personal observations and modified from (Rivas, 1953)). E. Schematic drawing of a sagittal section through the brain of the northern pike Esox lucius. The pineal vesicle is connected to the brain between the habenula (hc) and posterior (pc) commissures by the pineal stalk. The large pineal vesicle covers the cerebral hemispheres (ch) and olfactory bulbs (ob). The pineal lumen (pl), filled with cerebrospinal fluid, opens into the 3rd ventricle. ds, dorsal sac; on, optic nerve; vt, velum transversum. Modified from (Falcón et al., 2011). F. Eosin staining of a sagittal section through pineal area of the gilthead seabream, Sparus aurata. The pineal tissue is located in a pit, formed by the bone skull becoming substantially thinner in this area. G. Diagrammatic presentation of the 6 types of pineal epithelia in teleosts: 1, flat; 2: folded; 3, convoluted; 4, small space; 5, compact; 6, intermediate. Modified from (Omura & Oguri, 1969). © rightLinks Elsevier - Number 5903761097626
- Fig. 12. Pineal and retinal targets in the fish brain. The drawing is a schematic presentation of the pineal (red arrows) and optic (green arrows) nerve projections, together with the areas where the melatonin receptors have been identified (pink dots). A, anterior prethalamic nucleus; Cp, central posterior thalamic nucleus; Dp, dorsal posterior thalamic nucleus; DT, dorsal tegmental nucleus; Hb, habenula; NFLM, nucleus of the medial longitudinal fascicle; NG, nucleus glomerulosus; NLV, lateral nucleus of the valvula; OC, optic chiasm; OT, optic tectum; Pa, paracommissural pretectal nucleus; PC, posterior commissure; PPd, dorsal periventricular pretectal nucleus; POA, preoptic nucleus; RF, reticular formation; SCN, suprachiasmatic nucleus; TPp, periventricular nucleus of the posterior tubercle; Vm, trigeminal motor nucleus; TS, torus semicircularis; VT, ventral prethalamus. Data from (Yáñez et al., 2009 and Servili et al., 2011) for the pineal projections, and from (Herrera-Pérez et al., 2010; Mazurais et al., 1999, and Feng et al., 2019) for the melatonin receptors. © rightLinks Elsevier - Number 5903761097626
- Fig.14 : Schematic presentation of the photo- and thermo-periodic regulation of the brain-pituitary-gonadal (BPG) axis. The photoperiodic (retina and pineal) and photo-thermo-periodic (pineal) information reach the neuroendocrine axis through non-visual retinal and pineal nerve fibers (brown arrows) and pineal melatonin (red circles) released into the cerebrospinal fluid and circulation. Melatonin acts through specific receptors (purple boxes) found at all stages of the BPG axis. Deep brain thermoreceptors and photoreceptors might also operate in the basal telencephalon. Melatonin effects have been reported on the main preoptic (POA) and hypothalamic stimulatory (GnRH1/3, Kp2, ⊕) and inhibitory (GnIH, dopamine, ⊖) factors known to control fish pituitary gonadotropes (FSH and LH cells). At the pituitary level melatonin also modulates the production of adenohypophysial hormones, including those involved in the control of reproduction, FSH, LH and TSH. TSH from the pituitary may regulate FSH and LH productions via the pituitary folliculostellate cells and/or the brain deiodinase-2 (Dio2), which allows production of T3 (triiodothyronine) from T4 (thyroxine). FSH and LH act on the gonads (ovary and testis) to control the progress of gametogenesis and steroidogenesis. A feedback of gonadal steroids operates on the pineal gland, reproductive brain and pituitary (green dotted lines). Estradiol (E2) also acts on the liver of female fish to promote the synthesis and release of vitellogenin (Vg), which reaches the ovary through the vascular system and contributes to the progression of vitellogenesis. Moreover, nerve projections (colored dotted lines) from the habenula (hab), containing Kp1-producing cells, and mesencephalic tegmentum (teg), containing Gnrh2 and Gnih neurons, reach the fish pineal gland, whereas the retina receives Gnrh3 fibers originating from cells in the olfactory bulbs (ob). The saccus vasculosus (SV), present in some fish species, contains opsin proteins, Tsh and Dio2; it might also act as a sensor of seasonal changes in day length in some fish species. The pineal gland also produces AgRP2 (agouti-related peptide-2), although its function remains unknown. [⏱️] : circadian clock machinery. © rightLinks Elsevier - Number 5903761097626