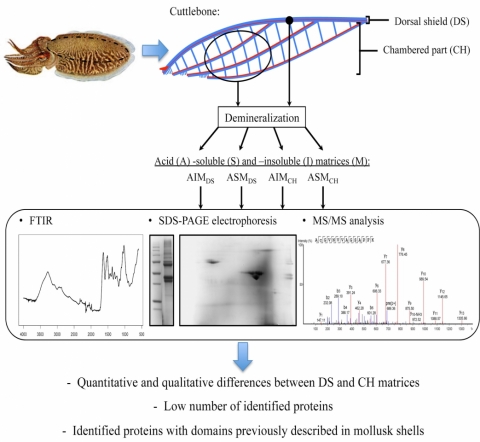
First proteomic analyses of the dorsal and ventral parts of the Sepia officinalis cuttlebone
Author(s): Charles Le Pabic, Arul Marie, Benjamin Marie, Aline Percot, Laure Bonnaud-Ponticelli, Pascal Jean Lopez, Gilles Luquet
Source: Journal of Proteomics (2017) 150: 63–73. doi: 10.1016/j.jprot.2016.08.015.
The cuttlefish's inner shell, better known under the name “cuttlebone”, is a complex organo-mineral structure, unique in mollusks and involved in tissue support and buoyancy regulation.
Although it combines useful properties as lightness, high compressive strength, high porosity and high permeability, knowledge of the organic compounds involved in its building remains limited.
Protein compounds constituting mollusk shells are known for their major roles in the biomineralization processes. These last years, a great diversity of shell proteins have been described in bivalves and gastropods allowing a better understanding of the calcification control by organic compounds and given promising applications in biotechnology
This paper analyzed for the first time the organic matrix of the aragonitic Sepia officinalis shell, with an emphasis on protein composition of two different structures: the dorsal shield and the chambered part. The results highlighted an organic matrix mainly composed of polysaccharides and (glyco-)proteins as previously described in other mollusk shells, but with quantitative and qualitative differences between the dorsal shield and the chamber part. Proteomic analysis resulted in identification of only a few protein compounds underlining the lack of reference databases for Sepiidae. However, most of them contain domains previously characterized in matrix proteins of aragonitic shell-builder mollusks, suggesting ancient and conserved mechanisms of the aragonite biomineralization processes within mollusks.
Contact: Charles Le Pabic (charles.lepabic@gmx.fr), Gilles Luquet, Team 1
