EMERGE - Environment, (epi)genoMEs, deteRminisms and ontoGEnesis
The team brings together colleagues from the unit specialized in ontogenesis processes, which are the processes that lead to the establishment of differentiated cells, tissues, organs or functional structures. These processes can occur during the development phases (embryo, larvae) as well as during the juveniles or adult phases: embryogenesis, morphogenesis, gametogenesis, gonadogenesis, neurogenesis and genesis of the shell (biomineralization). All these processes condition closely the adaptation of organisms to their environment and if their determinism is primarily genetic they are also likely influenced by their environment.
Members of the team develop their expertise on economically important species presenting relevant characteristics for the questions addressed: the differently structured germinal niches between oysters (Mollusc, bivalve) and the small-spotted catshark (Vertebrate, Chondrichthyes), gametogenesis blockings observed in triploid oysters with partial sterilisation, the particularly structured and developed nervous system of the common cuttlefish (Mollusc, Cephalopod) or even the biomineralization of ormers nacre (Mollusc, Gastropod). These species are located at key positions on the phylogeny, permitting the evolution history of ontogenesis processes studied to be adressed.
Questions adressed are the following:
>What are the cellular and molecular mechanism involved in the different ontogenesis processes studied?
>Does the environment impact these mechanisms and how?
The team’s researchers and engineers have an expertise in complementary approaches to address these different questions: chromosomic, genetic and epigenetic studies (qPCR, Next Generation Sequencing and bioinformatic, immunoprecipitation, karyotypes), cellular and histological studies (cellular cultures and sorting, qualitative and quantitative microscopy), molecular characterisation (immunolabeling, hybridisation), in vivo and in vitro functional tests (exposition, injections, transplantation, genome editing), experiments in controlled environments, fieldwork…
Axis 1: Molecular and cellular determinism of the studied ontogenesis processes
This axis decrypts the cellular and molecular pathways involved in the fate of cells engaged in the ontogenesis processes. Identity factors of stem cells (germinal, neural, somatic), factors participating in the different differentiation pathways possible (in gametes, neurons, supporting cells) or in the maintenance or even the blocking of their pluripotency state and factors driving the structures establishment and functioning (histogenesis and organogenesis, hormones, enzymes, neurotransmitters,…) will be studied more particularly.
Axis 2: Influence of environmental conditions on ontogenesis processes
The environmental impact can be particularly determining for the sexual determinism, in the gametogenesis initiation, the reproduction effort or the embryonic and larval development. Similarly, the nervous structure maturation is influenced by external stimuli and a process like shell mineralisation is necessarily dependent on the mineral availability in the environment. In the context of global changes, the environment can also be a disrupting source for ontogenesis: what is the impact of ocean acidification or temperature rising no shell development and morphogenesis? In which ways the changes in abiotic and biotic parameters can interfere in sexual maturation, sex ratios, cellular fate? What are the ontogenesis processes adaptation capacities or resilience?
Axis 3: Epigenetic and ontogenesis
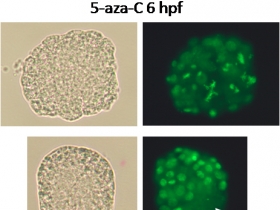
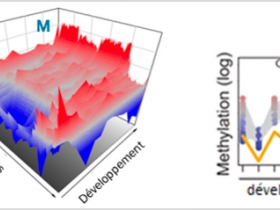
Indirect interactions exist between the environment and ontogenesis processes: via epigenetic mechanisms, the environment can modify and regulate genomes and transcriptomes and also imprint marks which will potentially be passed on from generation to generation. The oyster in particular, through its early development and reproduction control and its vulnerability to abiotic and biotic parameters, emerges as a lophotrochozoan model in this area.
Latest scientific articles
2024
-
. 2024. “Settlement Patterns And Temporal Successions Of Coral Reef Cryptic Communities Affect Diversity Assessments Using Autonomous Reef Monitoring Structures (Arms).”. Sci Rep 14 (1): 27061. doi:10.1038/s41598-024-76834-8.
-
. 2024. “Structural And Functional Characterization Of An Egg-Laying Hormone Signaling System In A Lophotrochozoan – The Pacific Oyster (Crassostrea Gigas)”. General And Comparative Endocrinology 346: 114417. doi:10.1016/j.ygcen.2023.114417. https://linkinghub.elsevier.com/retrieve/pii/S0016648023002228.
-
. 2024. “Mycorrhizal Communities Of Vanilla Planifolia In An Introduction Area (La Réunion) Under Varying Cultivation Practices”. Plants, People, Planet 6 (3): 683 - 696. doi:10.1002/ppp3.v6.310.1002/ppp3.10476. https://nph.onlinelibrary.wiley.com/doi/full/10.1002/ppp3.10476.
-
. 2024. “Ancestors’ Gift: Parental Early Exposure To The Environmentally Realistic Pesticide Mixture Drives Offspring Phenotype In A Larger Extent Than Direct Exposure In The Pacific Oyster, Crassostrea Gigas”. Environmental Science & Technology. doi:10.1021/acs.est.3c0820110. https://pubs.acs.org/doi/10.1021/acs.est.3c08201.
2023
-
. 2023. “M6A Profile Dynamics Indicates Regulation Of Oyster Development By M6A-Rna Epitranscriptomes”. Genomics, Proteomics & Bioinformatics 21 (4): 742 - 755. doi:10.1016/j.gpb.2022.12.002. https://linkinghub.elsevier.com/retrieve/pii/S1672022922001516.
-
. 2023. “Molecular And Phenotypic Effects Of Early Exposure To An Environmentally Relevant Pesticide Mixture In The Pacific Oyster, Crassostrea Gigas.”. Environmental Pollution 326: 121472. doi:10.1016/j.envpol.2023.121472. https://linkinghub.elsevier.com/retrieve/pii/S0269749123004748.
2021
-
. 2021. “Exploration Of Chemosensory Ionotropic Receptors In Cephalopods: The Ir25 Gene Is Expressed In The Olfactory Organs, Suckers, And Fins Of Sepia Officinalis.”. Chem Senses 46. doi:10.1093/chemse/bjab047.
-
. 2021. “Gonadal Transcriptomes Associated With Sex Phenotypes Provide Potential Male And Female Candidate Genes Of Sex Determination Or Early Differentiation In Crassostrea Gigas, A Sequential Hermaphrodite Mollusc.”. Bmc Genomics 22 (1): 609. doi:10.1186/s12864-021-07838-1.
-
. 2021. “Not Frozen In The Ice: Large And Dynamic Rearrangements In The Mitochondrial Genomes Of The Antarctic Fish”. Genome Biology And Evolution 13 (3). doi:10.1093/gbe/evab017. https://doi.org/10.1093/gbe/evab017.
-
. 2021. “Transcriptome Profiling Of The Pacific Oyster Crassostrea Gigas Visceral Ganglia Over A Reproduction Cycle Identifies Novel Regulatory Peptides”. Marine Drugs 19 (8): 452. doi:10.3390/md19080452. https://www.mdpi.com/1660-3397/19/8/452.
Team members
PhD Thesis
Programs
| 2020 to 2024 | PESTO |
| 2024 | NGSDISPER24 |
| 2024 | CRYPTOREEF2 |
| 2022 to 2024 | GenESis |
| 2023 | MIRO |
| 2023 | MitoPlancton |
| 2023 | NGSDISPER23 |
| 2022 | MOPHYBU |
| 2022 | ElasTique |
| 2018 to 2021 | ECUME |
| 2018 to 2021 | RaNTrans |
| 2019 to 2020 | Étude des propriétés physico-chimiques d’un produit marin à caractère industriel à base de gonades de cabillaud du genre Gadus |
| 2019 to 2020 | LET IT BI |
| 2019 to 2020 | HERITAGe |
| 2017 to 2020 | ICOBio |
| 2020 | OSIRIS |
| 2018 to 2019 | MATO |
| 2017 to 2019 | Salinité huitres |
| 2019 | CROCS (Comparaison des Récepteurs Olfactifs chez la Crevette et la Seiche) |
| 2018 to 2019 | ATM IMADO-2 |
| 2019 | TRICHO |
| 2016 to 2019 | Biligncell |
| 2017 to 2018 | ATM IMADO |
| 2017 to 2018 | ATM LOCUS |
| 2018 | Evolution des poissons cavernicoles dans la région karstique du Cerro Blanco |
| 2012 to 2016 | GIMEPEC |
| 2013 to 2016 | OLICEB |
| 2015 to 2016 | BestClim |
| 2013 to 2015 | TRIPLOIDES |
| 2014 to 2015 | ATM CRISPR |
| 2013 to 2015 | NAISSAIN |
| 2013 to 2014 | Buloclim |